The first batteries, most used and still common today, were lead-acid batteries, used in automobiles which could retain a good amount of energy for the basic purpose, to power a car, and also for other applications such as emergency lighting systems and even domestic energy production systems from alternative sources.
However, these batteries have barely evolved and their operating principle, as well as their storage capacity (energy density), has increased little over time. We can still use them, because they are easy to obtain, but little by little it is no longer the ideal solution.
With higher efficiency and higher energy density, NiCd batteries came to occupy a prominent place gradually. With more advanced technology, they have come to occupy a huge range of practical applications, from cell phones, phones, toys, tools and more, up to electric cars.
At an advanced stage of its evolution, more energy can be stored in less space. In addition, its cost falls day by day, as shown in the latest industry news.
However, storing energy through batteries still has some disadvantages, such as:
Storage is done by absorbing the energy which becomes part of the binding energy of the formed substance. In the same way that a gasoline molecule contains energy in the bonds of the carbon atoms with the hydrogen atoms, energy absorbed when the plant of the prehistoric ages stored it in its formation, from the solar energy and that later remained when the plant died and in millions of years it formed oil, in a battery we have a similar process.
The charge current causes a chemical reaction which causes the substance inside it to change (form a new substance) that retains the energy in the bonds of its atoms. When we connect the battery to a load, this substance delivers the stored energy in the form of an electric current.
This chemical process, in addition to being slow (the battery takes time to charge, as it is the time that the substance needs to change and store energy) has low performance.
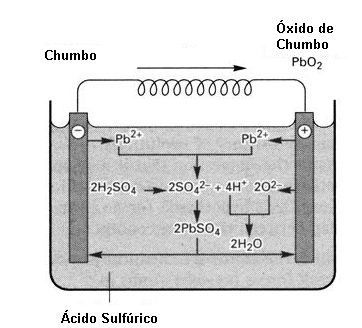
Batteries therefore consist of a chemical form of energy storage.
Another form, which until recently did not find practical use due to its low storage capacity, is that formed by capacitors.
A capacitor stores energy in the electric field which forms between its armatures when it is charged, as shown in Figure 3.
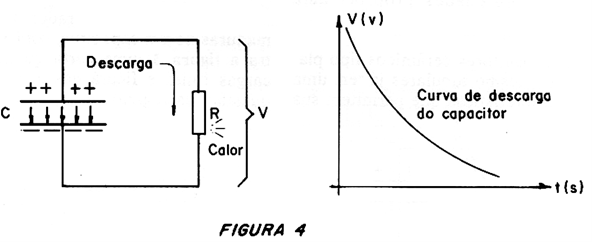
Its presence is manifested in the potential difference between the armatures, capable of producing a current when we connect this component to a load.
However, ordinary capacitors do not achieve reasonable energy storage, even the largest electrolytics.
This storage capacity, as we know, depends on the capacitance, which depends on the type of dielectric and its dimensions.
In recent years, however, based on nanotechnology, capacitors have been developed with enormous storage capacity, so large that they have started to compete with the best batteries and now even surpass them.
Supercapacitors can store as much energy as a regular battery and are already used in many important practical applications. In Figure 4, we have an electric Lamborghini car, which instead of the ordinary battery uses supercapacitors, providing power of more than 800 HP for its engine during a period that allows it to travel hundreds of kilometers.

Small supercapacitors with capacitances which are measured in Farads can be found at an affordable cost with ease. We remind you that 1 Farad is the capacitance of a spherical capacitor the size of Earth.

The advantages we get from storing energy in a supercapacitor are many. The first is the energy density, that is, storage capacity. We can store a lot of energy in a small space at a small weight.
The second factor, which is very important, is the charge mode. Not involving chemical processes, the load only depends on the source ability to supply energy. Thus, it has been heard of supercapacitors that would be used in electric cars that can be charged in 20 seconds. In the process, there is virtually no loss of energy.
Supercapacitors are becoming increasingly accessible and should be an excellent source of stored energy.
Inventiveness has no limit, and the need to obtain energy becomes increasingly greater in our times, when traditional sources are running out and we have more and more electrical equipment to serve us.
It is already said, for example, in dams that would have water pumped from lower levels to higher levels during the day, when solar cells can be fed and then, at night, water flowing through turbines would generate energy that would be available to everyone.
But a very interesting form was presented by an English company called LAES that intends to store electricity in tanks of liquid air.
LAES stands for Liquid Arir Energy Storage. Figure 6 shows a model of the power plant.
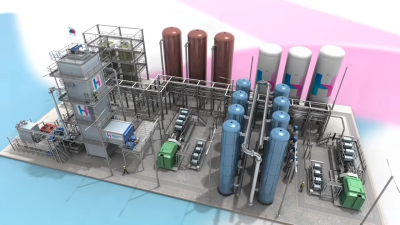
How does a power plant of this type work? Evidently, due to the size of the facilities, this is not a form of home energy.
You may have seen that when air escapes from a gas plug or gas installation, the exhaust location freezes, and the gas expands.
This indicates a heat exchange process: the heat from the environment is absorbed quickly, freezing the place and transferred to the expanding air or gas. In this expansion it can carry the energy it has absorbed, with an enormous capacity to supply energy.
Then see that we use energy to compress the gas. This energy is stored and then used for expansion. A practical installation for this type of power plant would not be very cheap and would occupy considerable space. Something to be considered for the future.




